Zoetis
BMD Soluble Powder
BMD Soluble Powder
Couldn't load pickup availability
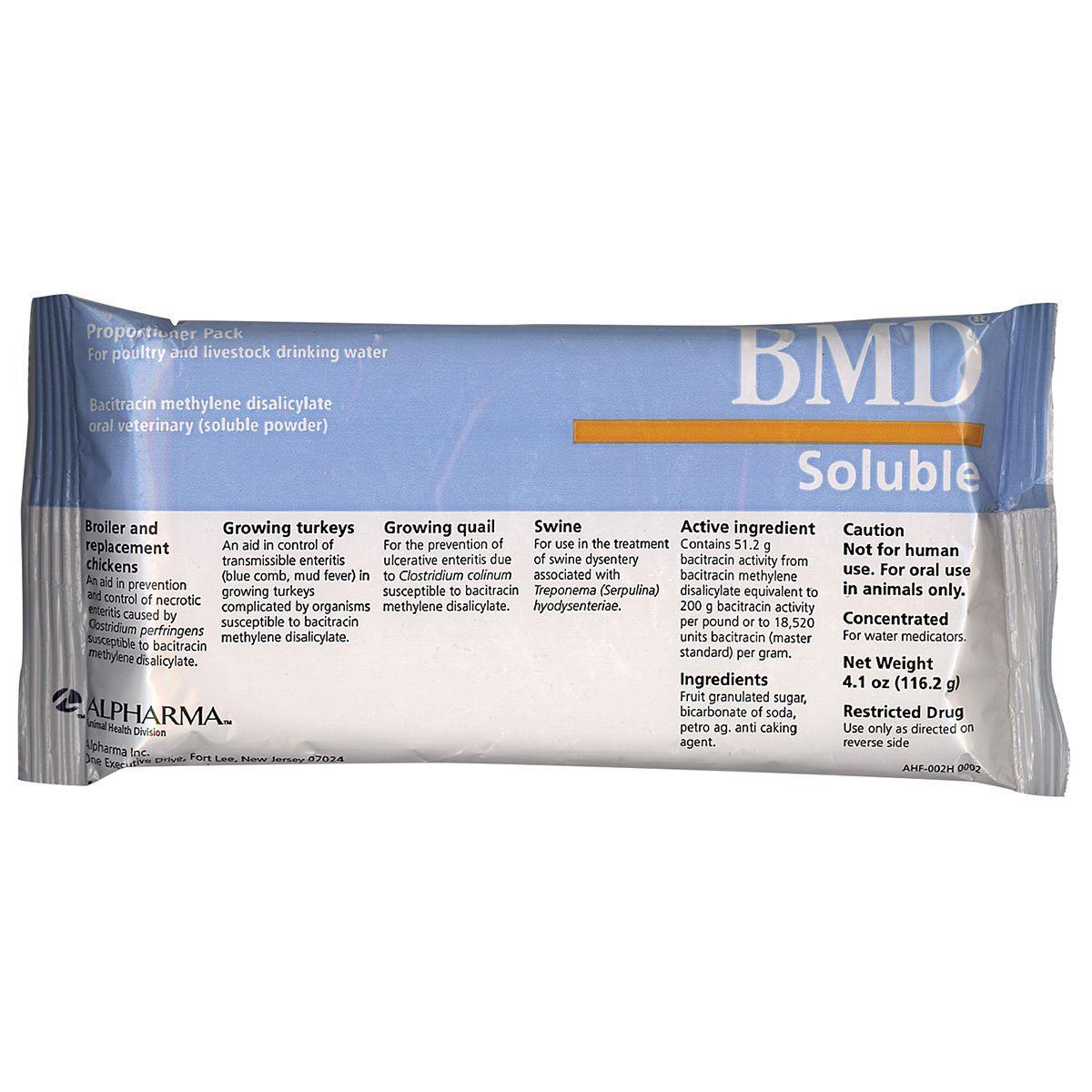
BMD Soluble Powder is an antibiotic powder which is added to the drinking water of poultry and livestock. Contains the active ingredient bacitracin from bacitracin methylene disalicylate.
Use in the treatment of swine dysentery associated with Brachyspira hyodysenteriae.
Use in broilers and replacement chickens as an aid in prevention of necrotic enteritis caused by Clostridium perfringens.
For growing turkey’s, use to control transmissible enteritis (blue comb, mud fever). Use in growing quail for the prevention of ulcerative enteritis caused by Clostridium colinum.
Soluble powder for poultry and livestock drinking water. Contains 51.2 g bacitracin activity from bacitracin methylene disalicylate. For use in the treatment of swine dysentery (bloody scours) associated with Treponema (Serpulina) hyodysenteriae.
Dosage: Swine-1000mg/gallon of water. Use as sole source of drinking water.
BMD Soluble Powder Mixing/Administration Instructions
For necrotic enteritis in broiler and replacement chickens: Administer at a rate of 100 mg per gal for prevention; and 200 to 400 mg per gal for control. Administer continuously 5 to 7 days or as long as clinical signs persist, then reduce medication to prevention levels (100 mg/gal).
For blue comb or mud fever in growing turkeys: Give 400 mg per gal for control. Administer continuously as long as clinical signs persist.
For the prevention of ulcerative enteritis in growing quail: Give 400 mg per gal.
For the control of swine dysentery: Give 1,000 mg per gal. Start medication at first signs of the disease or at time of exposure and administer continuously for 7 days or until signs of dysentery disappear.
Treatment is not to exceed 14 days.
Directions for use
For proportioners: Select the treatment dosage. Set the proportioner at the desired delivery rate. To prepare the proportioner’s stock solution, place the indicated quantity of BMD Soluble in a 2-gallon container, fill with water and stir until dissolved.
For a proportioner set of 1 oz/gal: Use 1/2 pack for a treatment dose of 100 mg/gal; use 1 pack for 200 mg/gal; use 2 packs for 400 mg/gal; use 5 packs for 1,000 mg/gal.
For a proportioner set at 2 oz/gal: Use 1/4 pack for a treatment dose of 100 mg/gal; use 1/2 pack for 200 mg/gal; use 1 pack for 400 mg/gal; use 2-1/2 packs for 1,000 mg/gal.
For tanks: 1 package will medicate about 50 gallons of water at a dosage rate of 1,000 mg/gal.
To control an outbreak: Start medication at the first clinical signs of the disease. Consult a diagnostic laboratory or veterinarian to determine the diagnosis and advice regarding the optimal level of drug.
Size: 4.1 oz
This item cannot be shipped to California.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us