Merck Animal Health
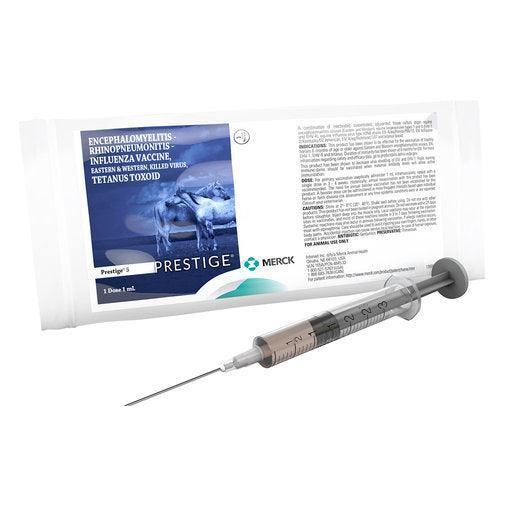
Prestige 5
Prestige 5
Couldn't load pickup availability
Prestige 5 is for the vaccination of healthy horses 6 months of age or older against Eastern and Western encephalomyelitis viruses, EIV, EHV-1, EHV-4 and tetanus. Duration of immunity has been shown at 6 months for EIV. Shown to decrease virus shedding of EIV and EHV-1. Foals nursing immune-dams should be vaccinated when maternal antibody levels will allow active immunization.
Combination of inactivated, concentrated, adjuvanted, tissue culture origin equine encephalomyelitis viruses (Eastern and Western), equine herpesviruses types 1 and 4 (EHV-1 and EHV-4), equine influenza virus type H3N8 strains EIV A/eq/Florida/RW/13, EIV A/Equine 2/Kentucky/02 American, EIV A/eq/Richmond/1/07 and tetanus toxoid.
Dosage: 1 ml IM, repeat in 3-4 weeks. Revaccinate annually.
Please see shipping information regarding vaccines before placing order. This item cannot ship to AA AE AP MN addresses
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us