Zoetis
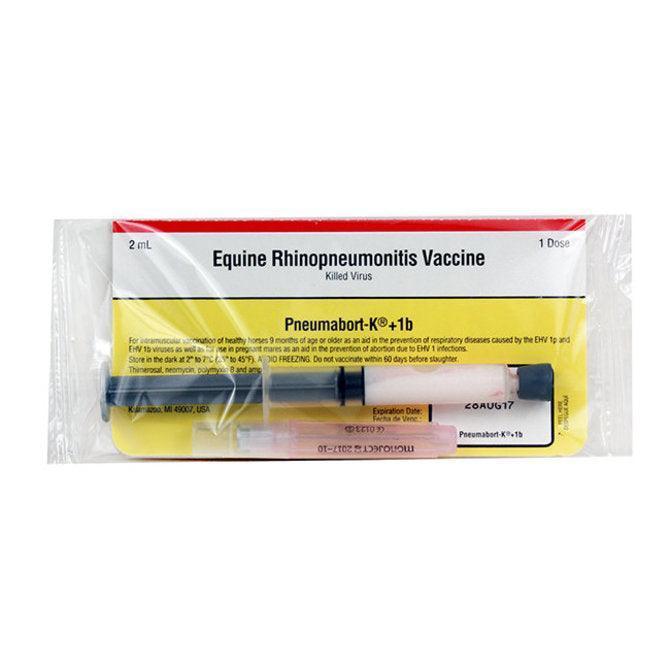
Pneumabort-K+1b
Pneumabort-K+1b
Couldn't load pickup availability
Please see shipping information regarding vaccines before placing order.
PNEUMABORT-K + 1b is the only equine vaccine labeled for use in pregnant mares to aid in the prevention of abortion due to equine herpesvirus type 1 (EHV-1) infections, as well as to help prevent respiratory infections caused by EHV-1p and EHV-1b. In the fifth, seventh and ninth months of gestation mares need a vaccine labeled to help protect against abortion caused by EHV-1. In fact, pregnant mares have a more than four times greater risk of abortion due to EHV-1 when not vaccinated.
Caution:
Store in the dark at 2° to 7°C (35° to 45°F). AVOID FREEZING. SHAKE VIGOROUSLY to assure uniform suspension of the vaccine. When used according to instructions, local or systemic reactions rarely occur. An occasional local swelling or induration at the site of injection may be encountered. Do not vaccinate within 60 days before slaughter. Use entire contents when first opened. In case of anaphylactoid reaction administer epinephrine.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us