Boehringer Ingelheim
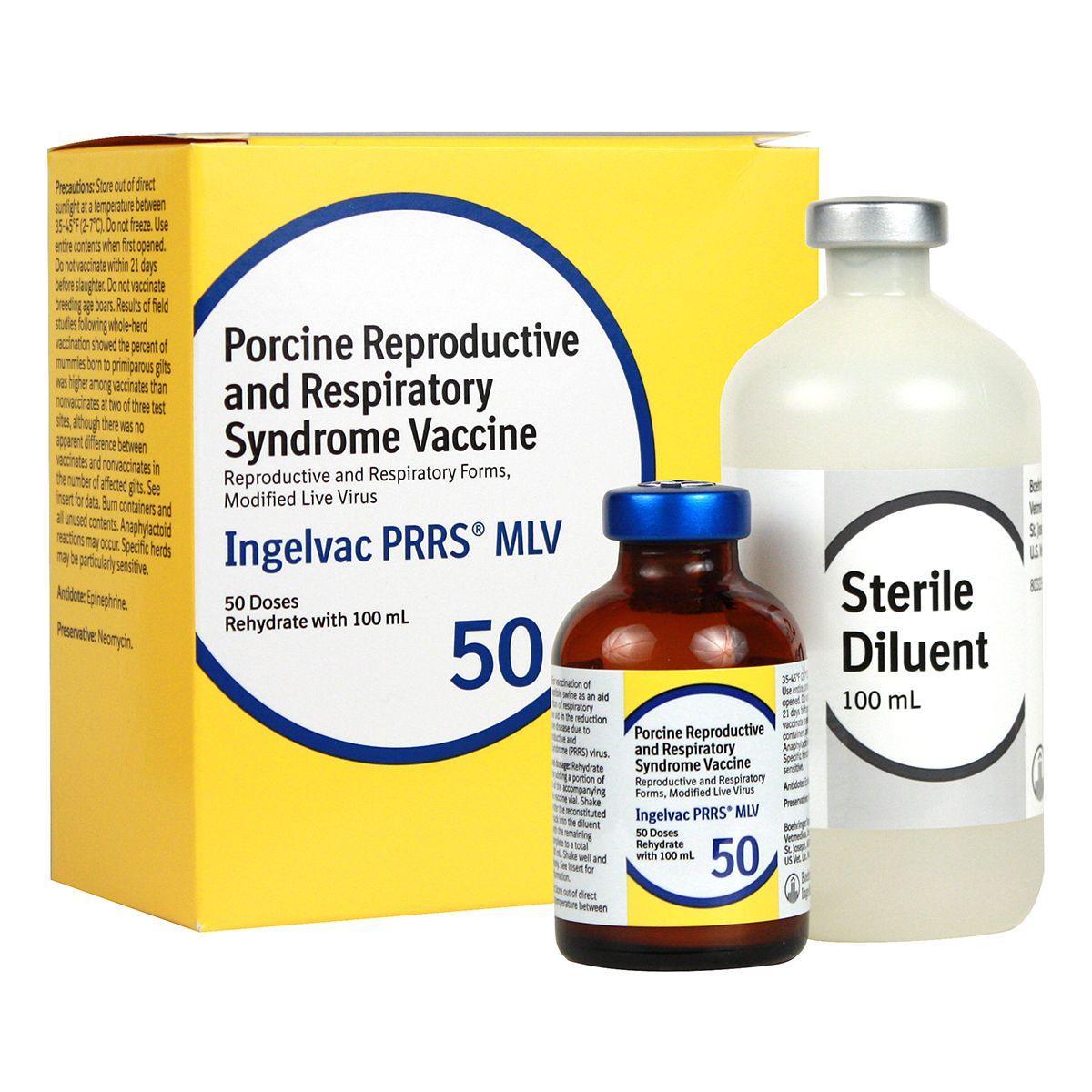
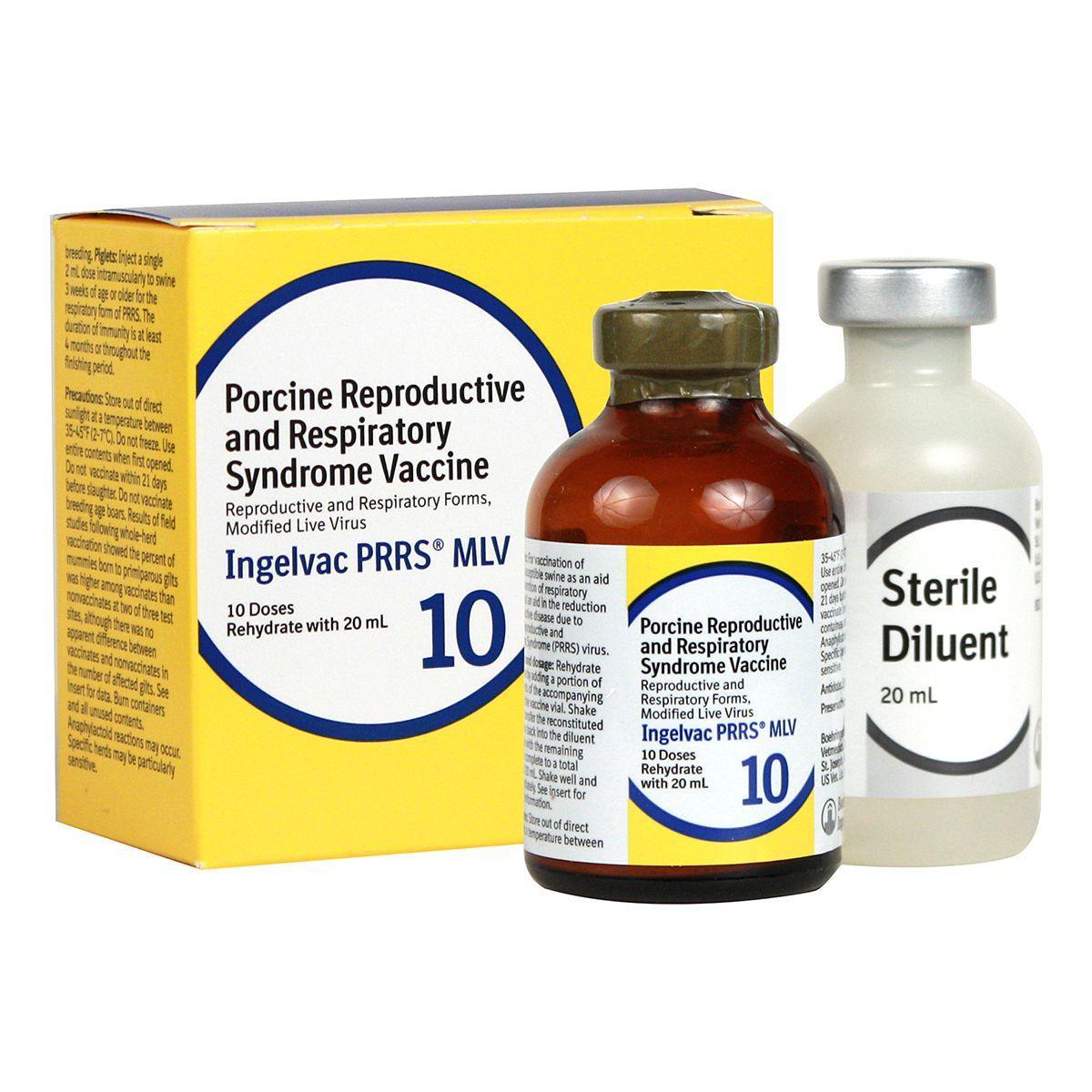
Ingelvac PRRS MLV
Ingelvac PRRS MLV
Regular price
$53.48 USD
Regular price
Sale price
$53.48 USD
Unit price
per
Shipping calculated at checkout.
Couldn't load pickup availability
Ingelvac PRRS MLV is recommended for use in healthy, susceptible swine in PRRS virus-positive herds only. For vaccination as an aid in the prevention of respiratory disease. Also aids in the reduction of reproductive disease due to PRRS virus.
Dosage:
- Piglets - 2 ml IM to pigs 3 weeks of age or older for the respiratory form of PRRS
- Sows and gilts - 2 ml IM for the reproductive form of PRRS.
Vaccination can either be population-targeted as a whole-herd vaccination program where all pregnant and non-pregnant sows and gilts are vaccinated every 3-4 months, or individual animal-targeted by vaccination 3-4 weeks prior to each breeding. Not for use in breeding age boars. 21-day slaughter withdrawal.
Please see shipping information regarding vaccines before placing order.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us