Boehringer Ingelheim
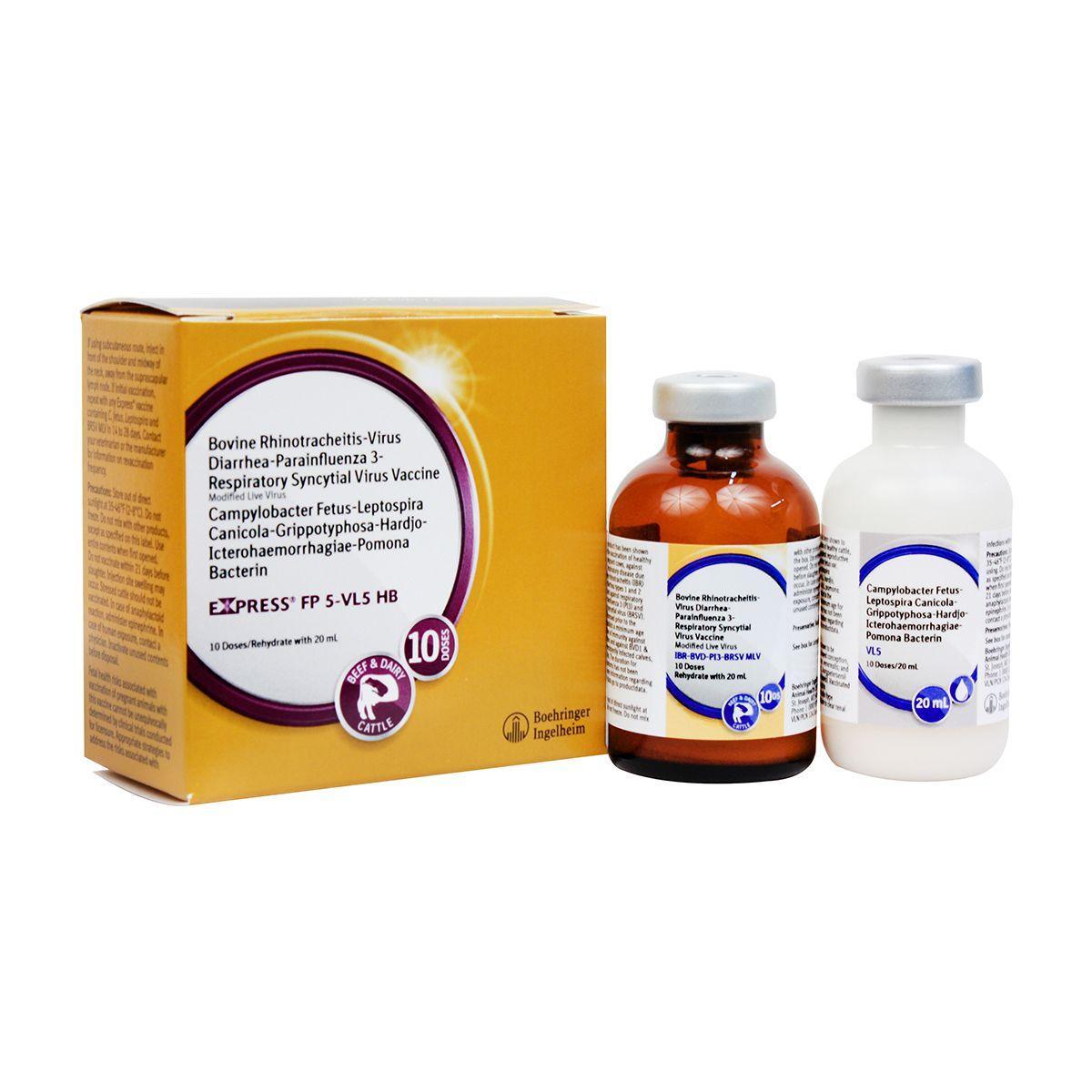
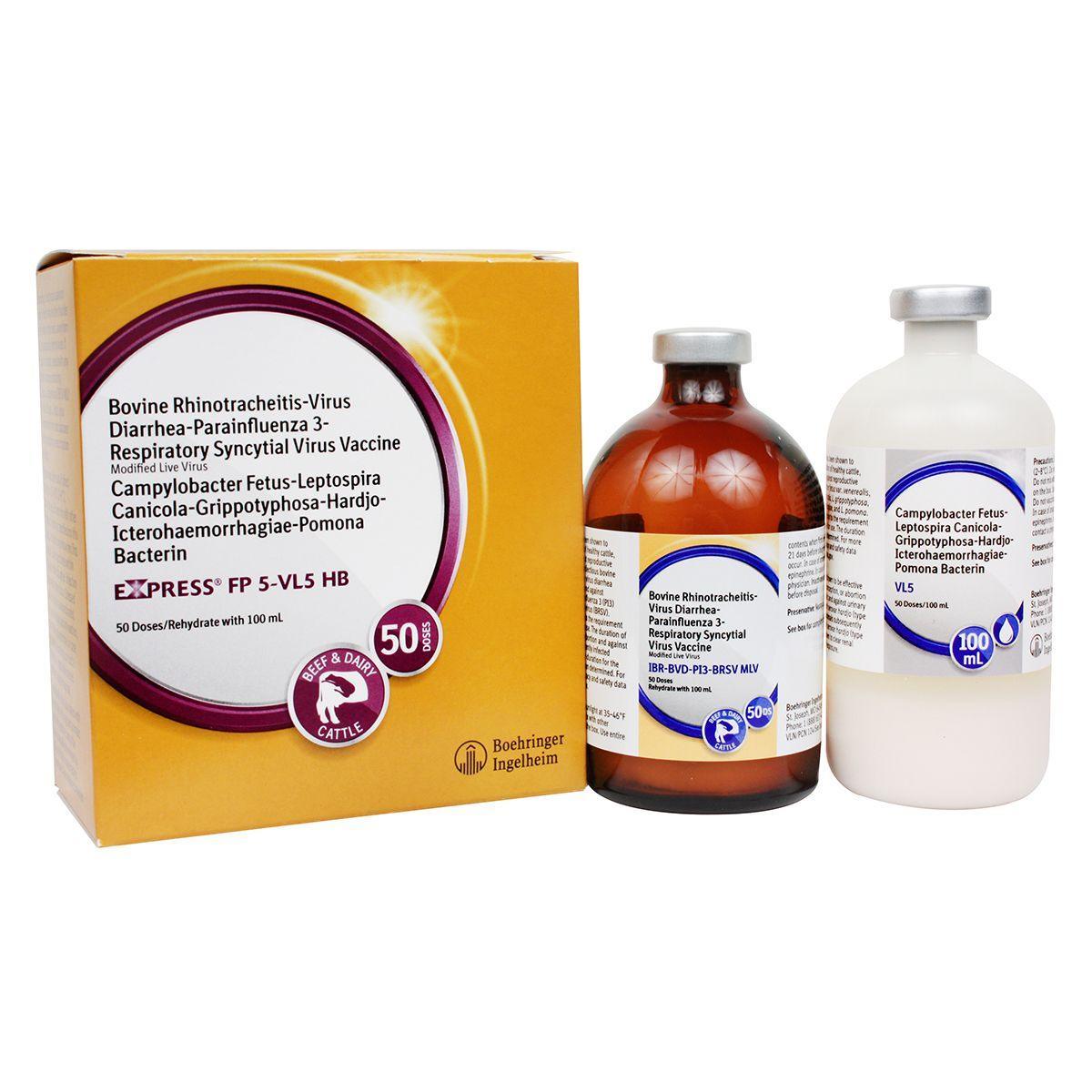
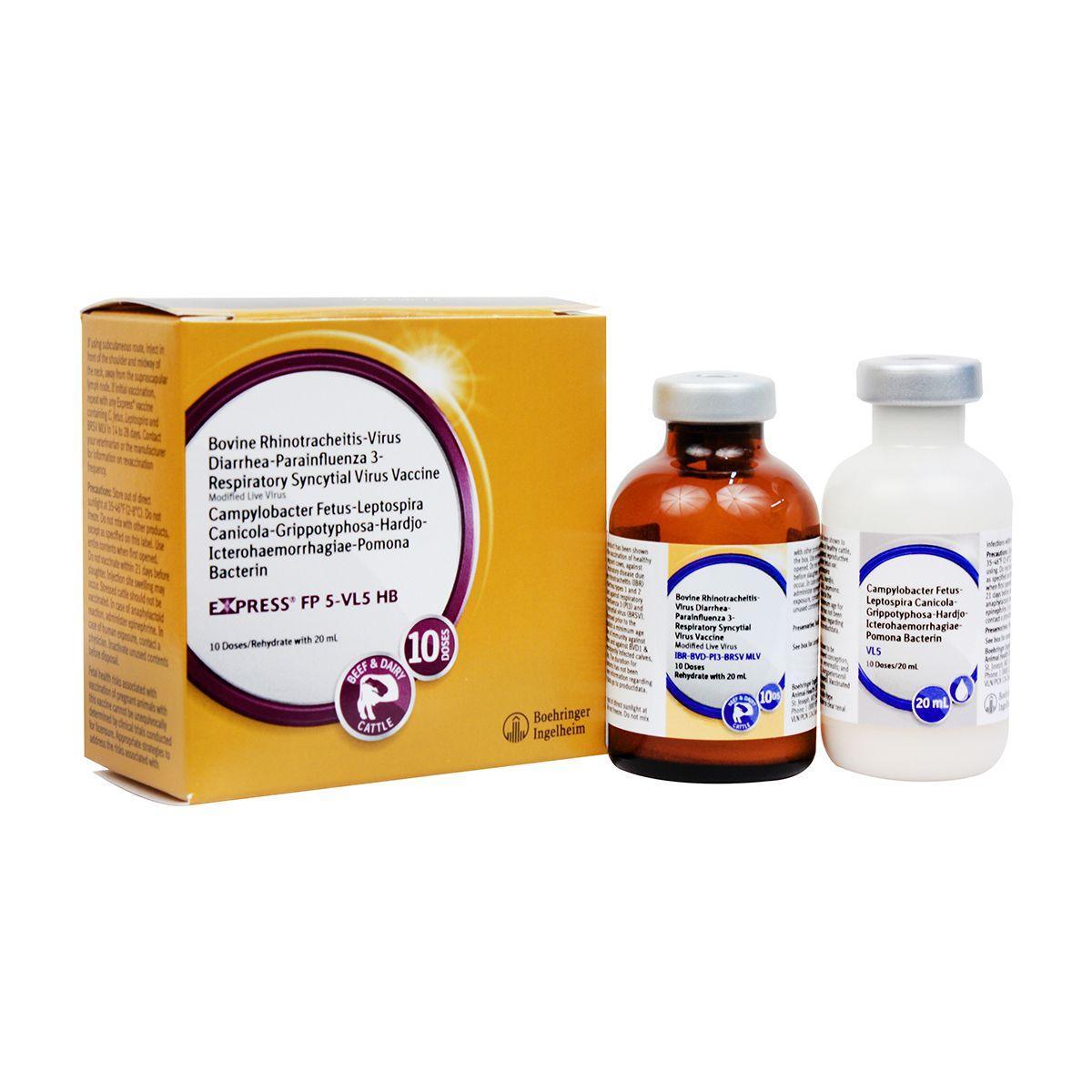
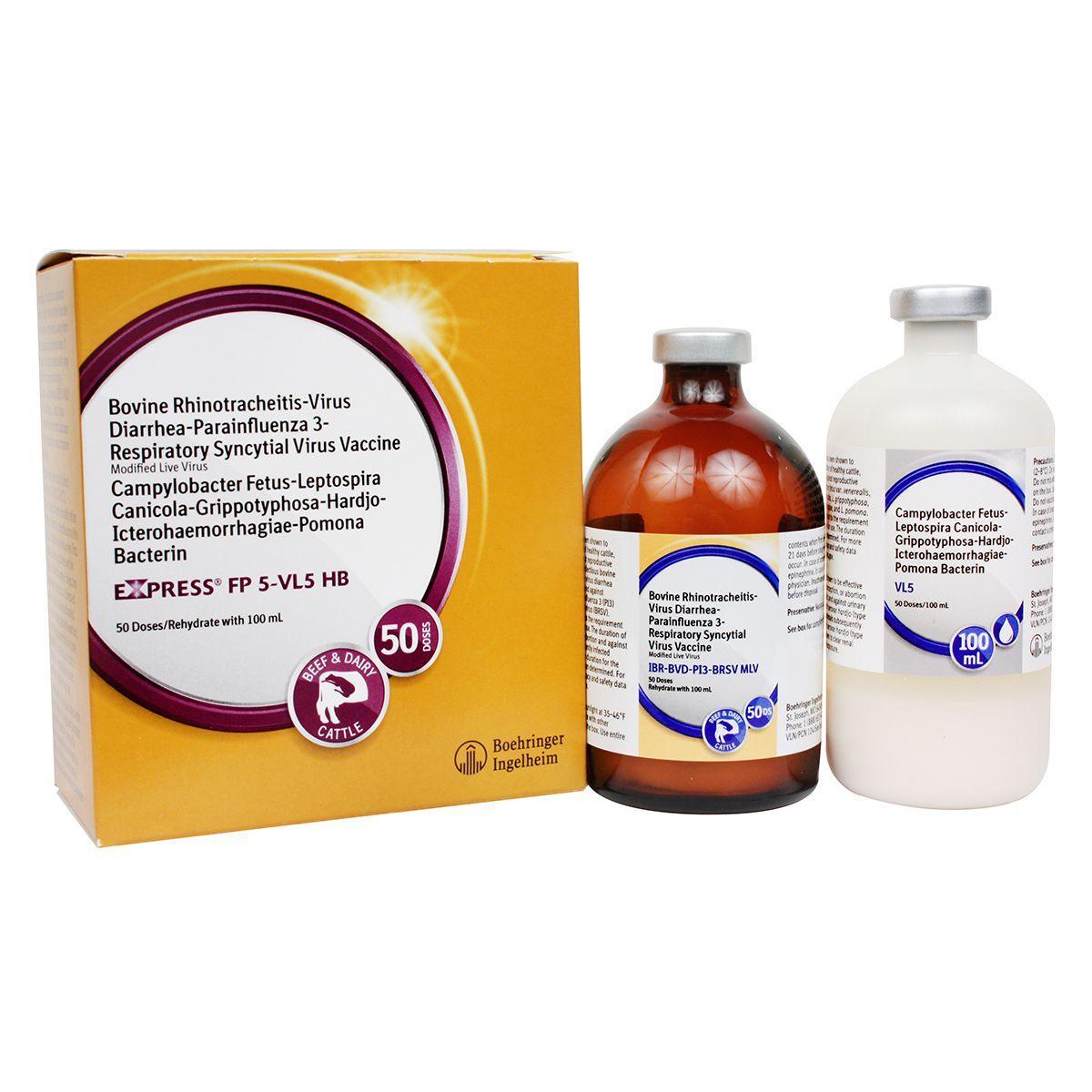
Express FP 5-VL5 HB
Express FP 5-VL5 HB
Couldn't load pickup availability
Please see shipping information regarding vaccines before placing order.
Modified-live virus for vaccination of healthy cows and heifers prior to breeding for prevention of persistently infected calves caused by BVD Types 1&2, including Type 1b; and as an aid in prevention of abortion due to IBR; for prevention of urinary shedding of L. hardjo-bovis; as an aid in prevention of respiratory disease caused by IBR, BVD Types 1&2 and BRSV; as an aid in reduction of respiratory disease caused by PI3, and as an aid in reduction of infertility, delayed conception or abortion caused by Campylobacter fetus var. venerealis and leptospirosis caused by the 5 most common strains of Lepto. IBR, BVD Types I and II, PI3, BRSV, Vibrio and 5 strains of Lepto including hardjo-bovis. Give 2 ml SQ or IM. For primary vaccination, give about 4 weeks prior to breeding and booster in 14-28 days. Safe for pregnant cows or calves nursing pregnant cows, providing they were vaccinated pre-breeding according to label directions with any Express® FP 5-VL5 HB vaccine. Calves vaccinated before 6 months of age should be revaccinated at 6 months. Annual booster is recommended.
Precautions
Store out of direct sunlight at 35-46°F (2-8°C). Do not freeze. Do not mix with other products, except as specified on the label. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Injection site swelling may occur. Stressed cattle should not be vaccinated. In case of anaphylactoid reaction, administer epinephrine. In case of human exposure, contact a physician. Inactivate unused contents before disposal. Fetal health risks associated with vaccination of pregnant animals with this vaccine cannot be unequivocally determined by clinical trials conducted for licensure. Appropriate strategies to address the risks associated with vaccine use in pregnant animals should be discussed with a veterinarian. No vaccine can be expected to have 100% efficacy under all conditions. A small number of calves persistently infected with BVD may have a devastating effect on herd health.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us