Boehringer Ingelheim
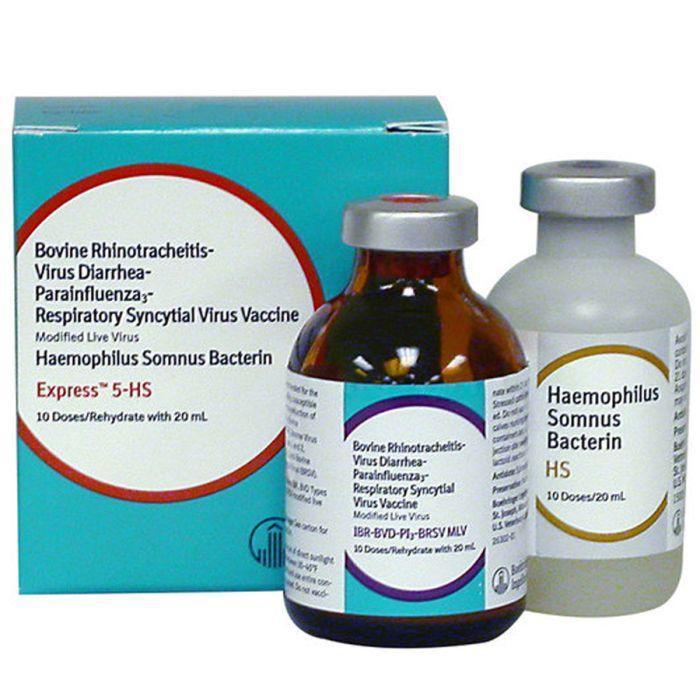
Express 5-HS
Express 5-HS
Couldn't load pickup availability
Please see shipping information regarding vaccines before placing order.
Express® 5- HS - 10 Dose is for the vaccination of healthy, susceptible cattle as an aid in the reduction of respiratory diseases caused by Bovine Rhinotracheitis (IBR) virus, Bovine Virus Diarrhea (BVD) Types 1 and 2, Parainfluenza 3 (PI3) virus, and Bovine Respiratory Syncytial Virus (BRSV), and as an aid in the prevention of disease caused by Haemophilus somnus. This vaccine may be used in pregnant females or calves nursing pregnant females, provided the females were vaccinated pre-breeding according to label directions with any Express® FP vaccine. Modified-live virus for vaccination of healthy, susceptible cattle as an aid in prevention of respiratory disease caused by IBR, BVD Types 1 and 2, and BRSV; as an aid in reduction of respiratory disease caused by PI3; and as an aid in prevention of disease caused by Haemophilus somnus.
Precautions
Store out of direct sunlight at 35-46°F (2-8°C). Do not freeze. Do not mix with other products, except as specified on the label. Use entire contents when first opened. Do not vaccinate within 21 days before slaughter. Injection site swelling may occur. Stressed cattle should not be vaccinated. In case of anaphylactoid reaction, administer epinephrine. In case of human exposure, contact a physician. Inactivate unused contents before disposal.
Fetal health risks associated with vaccination of pregnant animals with this vaccine cannot be unequivocally determined by clinical trials conducted for licensure. Appropriate strategies to address the risks associated with vaccine use in pregnant animals should be discussed with a veterinarian. No vaccine can be expected to have 100% efficacy under all conditions. A small number of calves persistently infected with BVD may have a devastating effect on herd health.
Dosage: 2 ml subcut. If initial vaccination, repeat with any Express vaccine containing BRSV modified-live virus and H. somnus in 14-28 days. Calves vaccinated before 6 months of age should be revaccinated at 6 months. Revaccinate annually. May be used in pregnant females or calves nursing pregnant females, provided the females were vaccinated pre-breeding according to label directions with any Express FP vaccine. 21 day slaughter withdrawal.
Note: It is possible that healthy-appearing cattle can be persistently infected with or incubating virulent BVD virus at the time of vaccination. In view of these findings and suggested causes, BVD vaccine is contraindicated in persistently infected cattle and use should be limited only to healthy, immunocompetent, unstressed cattle.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us