Boehringer Ingelheim
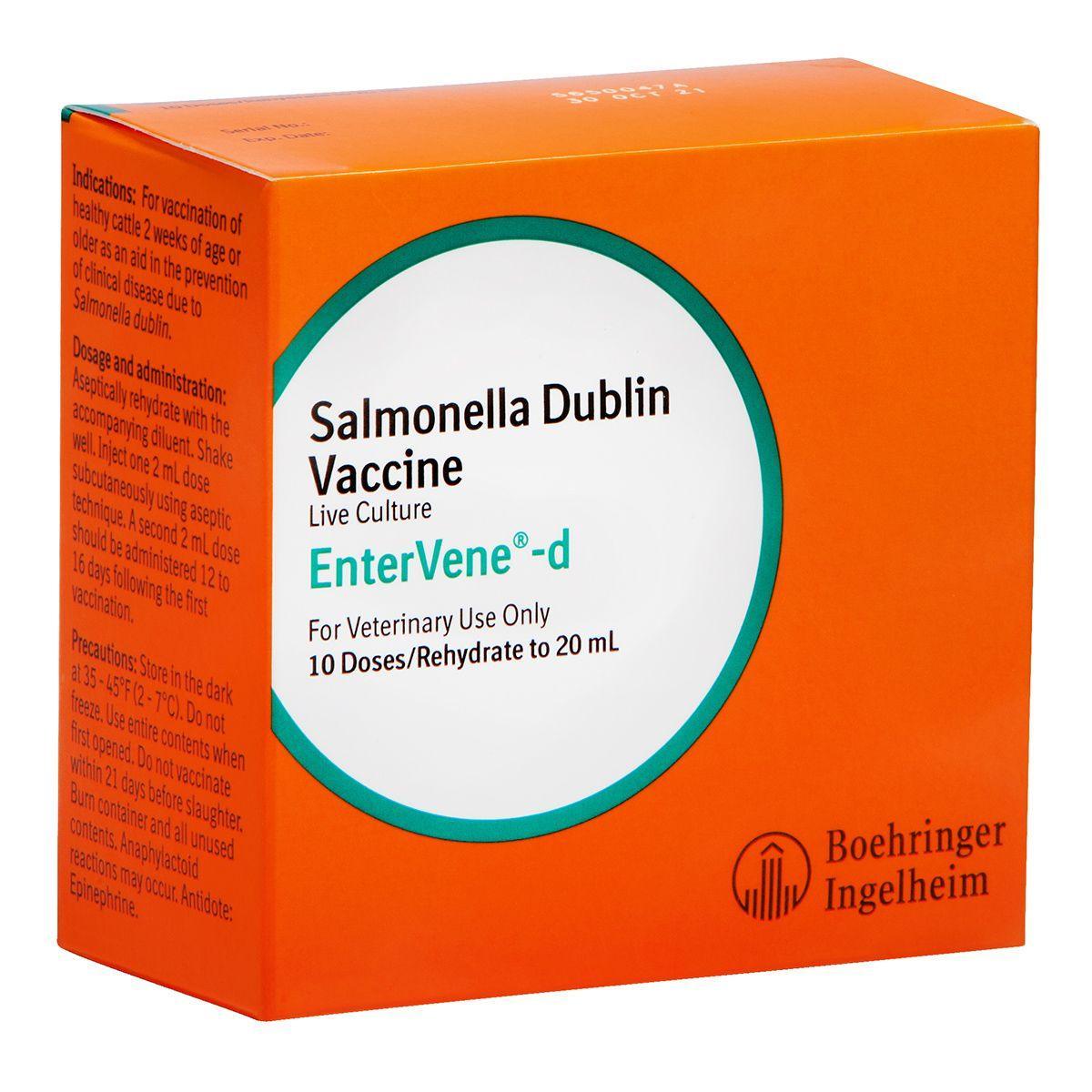
EnterVene-d
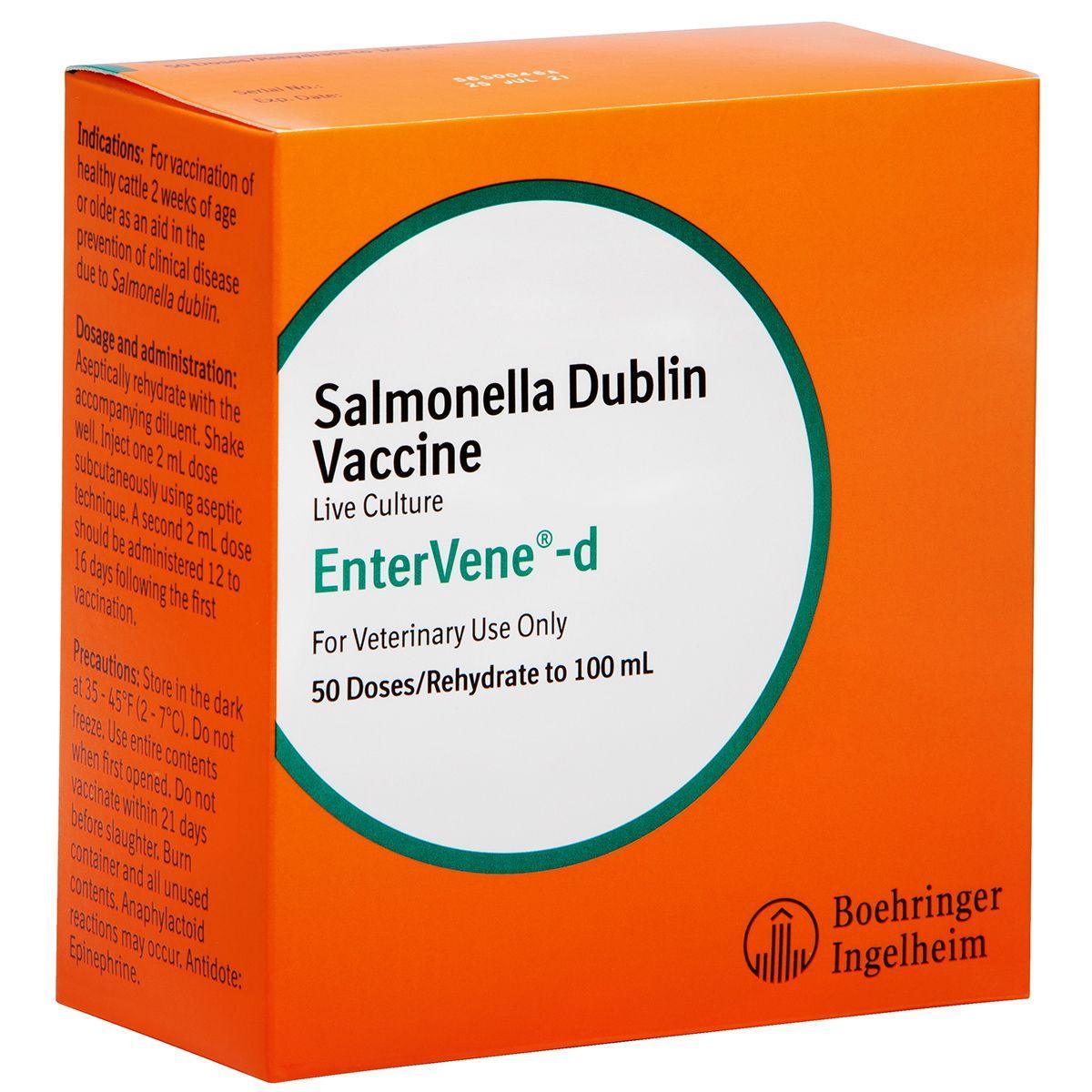
EnterVene-d
Couldn't load pickup availability
Please see shipping information regarding vaccines before placing order.
For the vaccination of healthy calves, two weeks of age and older, as an aid in the prevention of disease caused by salmonella Dublin. Proven to reduce mortality and clinical signs in calves exposed this strain of salmonella. Live culture vaccine that requires mixing.
Dosage: Inject 2 mL dose SQ using aseptic technique. A second 2 mL dose should be administered 12 to 16 days following the first vaccination. 21 day slaughter withhold.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us