Aspen Vet
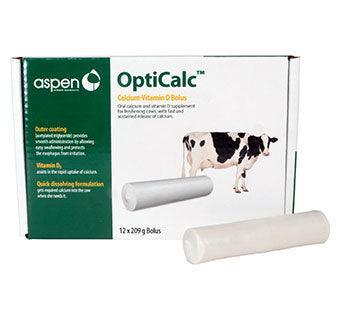
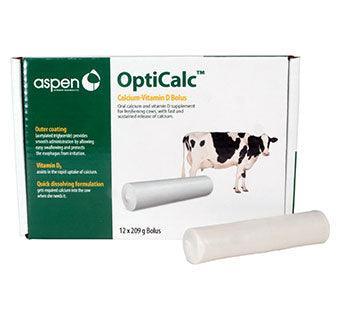
Aspen Vet OptiCalc Calcium Bolus
Aspen Vet OptiCalc Calcium Bolus
Couldn't load pickup availability
Aspen Vet OptiCalc Calcium Bolus is a supplemental source of calcium for dairy during the demanding period at freshening. Contains immediate-release calcium and sustained-release calcium plus vitamin D which is necessary for the absorption and metabolism of calcium and phosphorus.
▪Outer coating (acetylated triglyceride) provides smooth administration
by allowing easy swallowing and protects the esophagus from irritation
▪Vitamin D3 assists in the rapid uptake of calcium
▪Quick dissolving formulation gets required calcium into the cow when
she needs it
Pack of 12 boluses
Dosage and Administration:
- Minimum Protocol:
- Give one OptiCalc Calcium Bolus immediately after calving and a second bolus 12-24 hours after calving
- Administer using a bolus applicator
Extended Protocol:
For high yielding administration:
Discuss protocol with your veterinarian prior to implementation.
- 1st bolus at the first sign of parturition
- 2nd bolus immediately after calving
- 3rd bolus 12-24 hours after calving
- 4th bolus 24-48 hours after calving
Administration of Bolus:
- Remove packaging and place the bolus in the bolus applicator with the rounded end pointing outward
- Hold the cow’s upper jaw with your left hand and keep the cow’s head at a natural position and a little towards yourself
- Gently guide the loaded applicator into the cow’s mouth
- When you feel resistance, gently guide the applicator over the tongue
- When the applicator is behind the tongue, squeeze the handle to release the bolus
FOR ANIMAL USE ONLY. KEEP OUT OF REACH OF CHILDREN.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us