Boehringer Ingelheim
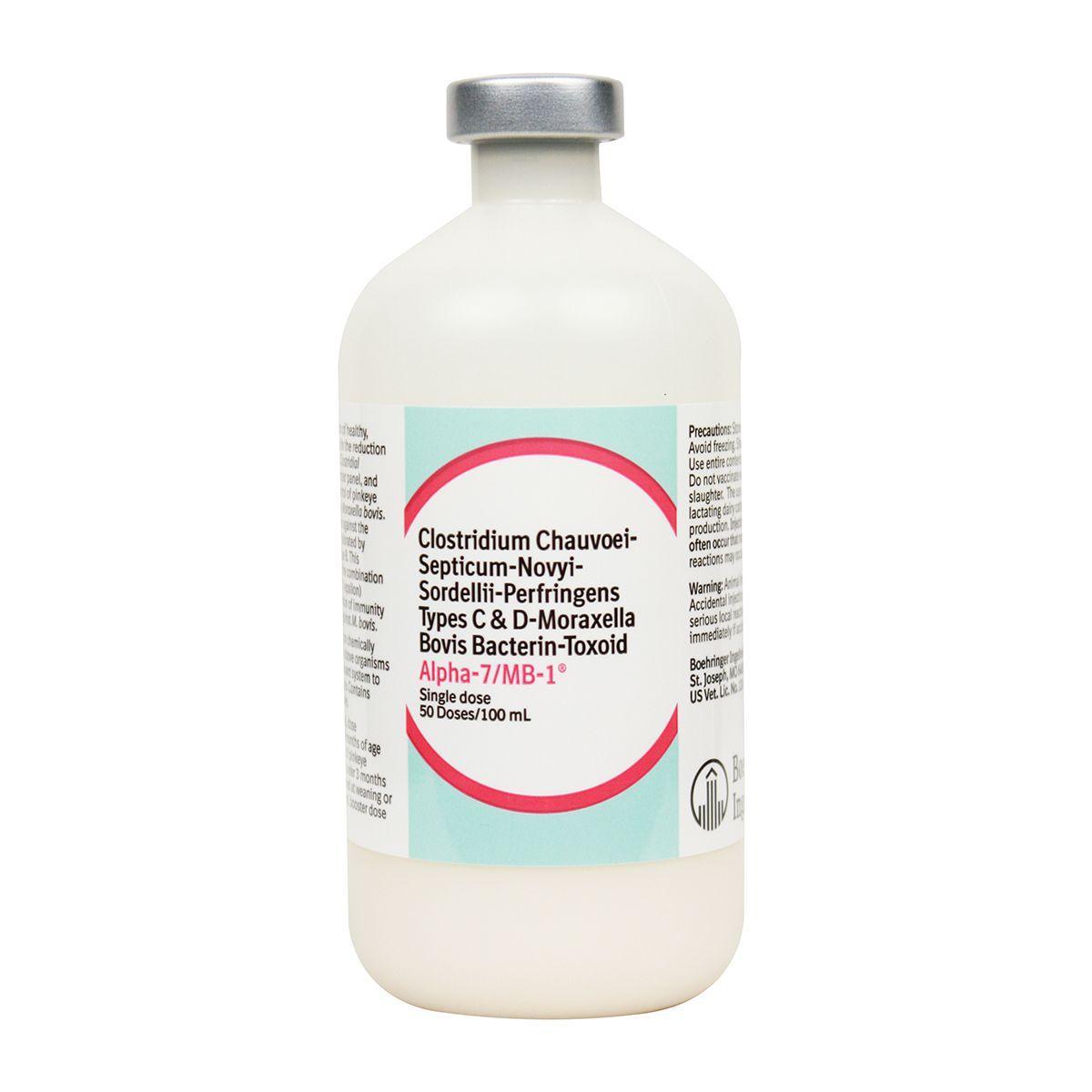
Alpha-7/MB-1
Alpha-7/MB-1
Couldn't load pickup availability
Please see shipping information regarding vaccines before placing order.
Bacterin-toxoid recommended for vaccination of healthy, susceptible cattle as an aid in the reduction of diseases caused by 7 types of Clostridial organisms and Pinkeye. Contains chemically inactivated organisms with special DD-2TM adjuvant system to maximize immune response.
Dosage: 2 ml subcut to cattle 2 months of age or older prior to the onset of pinkeye season. Calves vaccinated under 3 months of age should be revaccinated at weaning or 4-6 months. Revaccinate annually. May cause reduced milk production in lactating dairy cattle. 60 day slaughter withdrawal.
The Boehringer Ingelheim Vetmedica, Inc. Range Ready program is designed to assist seed stock producers manage their risk through a transferable limited health warranty on qualifying young bulls and replacement heifers enrolled in the program.
Manufacturer and/or Label Information
Manufacturer and/or Label Information
Dimensions
Dimensions
Care information
Care information
Let customers speak for us